

Now lets talk about how Oracle Argus Safety is able to achieve the above: Oracle Argus Safety – Ensures Global Regulatory compliance
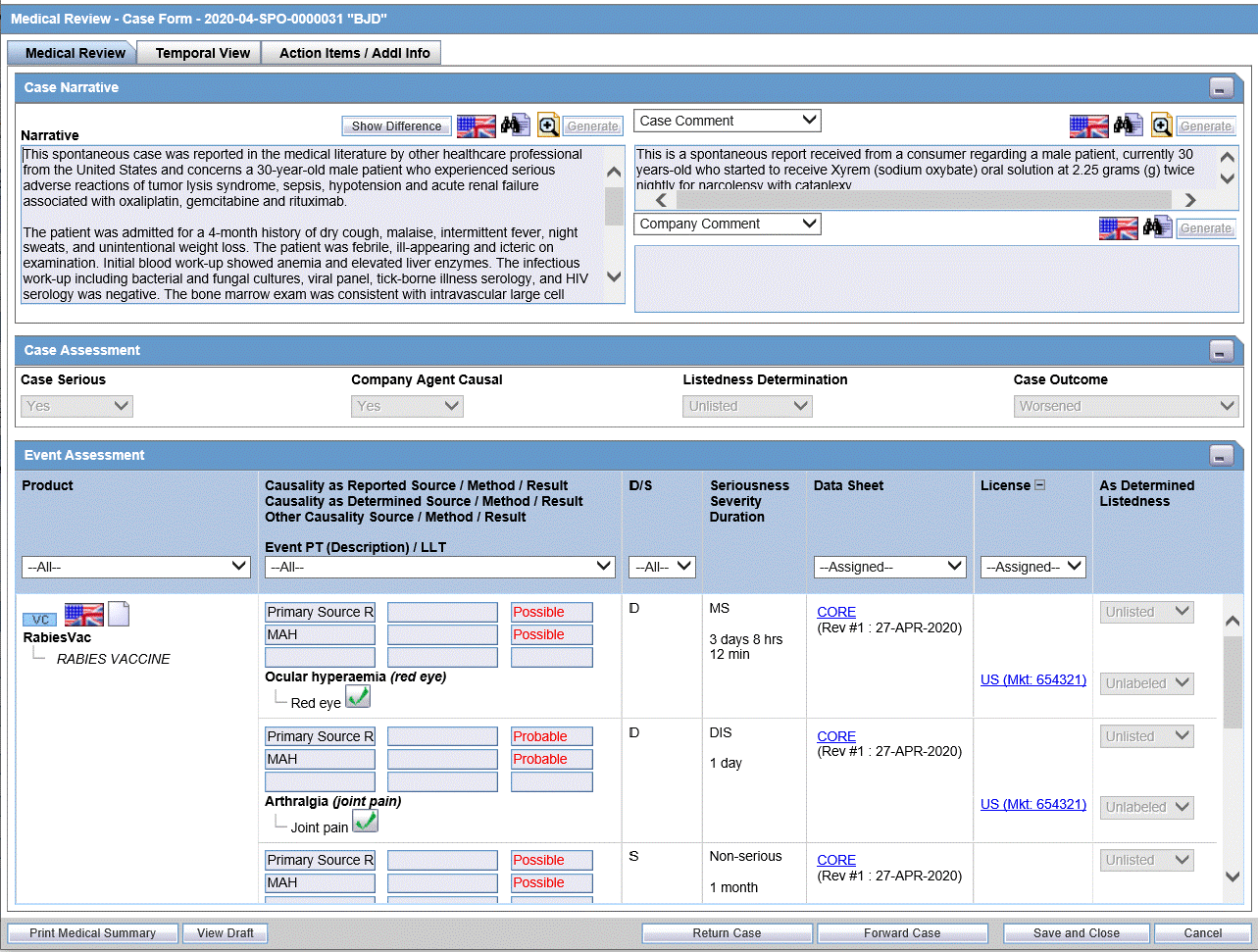
Firstly let us discuss in detail about the Oracle Argus Safety software, which is the de-facto standard for management of drug safety data used in the industry today.
ARGUS DEVELOPER TRIAL VERSION SOFTWARE
We shall discuss some most commonly used software in Pharmacovigilance as we go further in this post. WebVDME Pharmacovigilance Signal detection and Signal management software.Oracle Adverse Event Reporting System (AERS).Some software’s used in pharmacovigilance are: Let’s take a look at some software used in Pharmacovigilance for the management and reporting of Adverse events. Post marketing surveillance uses tools such as data mining of spontaneous reporting systems and patient registries, and investigation of case reports to identify the relationship between drugs and ADRs. Even very severe ADRs are often undetected because study populations are small. Because clinical trials involve several thousand patients at most less common side effects and ADRs are often unknown at the time a drug enters the market. Pharmacovigilance is gaining importance for doctors and scientists as the number of stories in the mass media of drug recalls increases. “A response to a drug which is noxious and unintended, and which occurs as doses normally used…for the prophylaxis, diagnosis, of therapy of disease, or for the modification of physiological function.” Pharmacovigilance is particularly concerned with adverse drug reactions, or ADRs, which are officially described as: The process of collection of such information about a drug begins in Phase I of the clinical trial, before approval of the drug, and continues even after approval several post-market safety studies are conducted, with many made mandatory by drug regulatory agencies around the world. Pharmacovigilance starts from the clinical stage and continues throughout the product life cycle of the drug, mainly divided as pharmacovigilance during pre-marketing (that is clinical phase) and post-marketing. Identifying new information about hazards associated with medicines.Generally speaking Pharmacovigilance is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and patients on the adverse effects of medications, biological products, herbalism and traditional medicines with a view to: Pharmacovigilance is the pharmacological science relating to the detection, assessment, understanding and prevention of adverse effects, particularly long term and short term side effects of medicines.



 0 kommentar(er)
0 kommentar(er)
